The number of electrons in per shell of selenium atom is 2 8 18 6. A valence electron is an outer shell electron and may participate in the formation of a chemical bond.

Valence Electrons Configuration Of All Elements
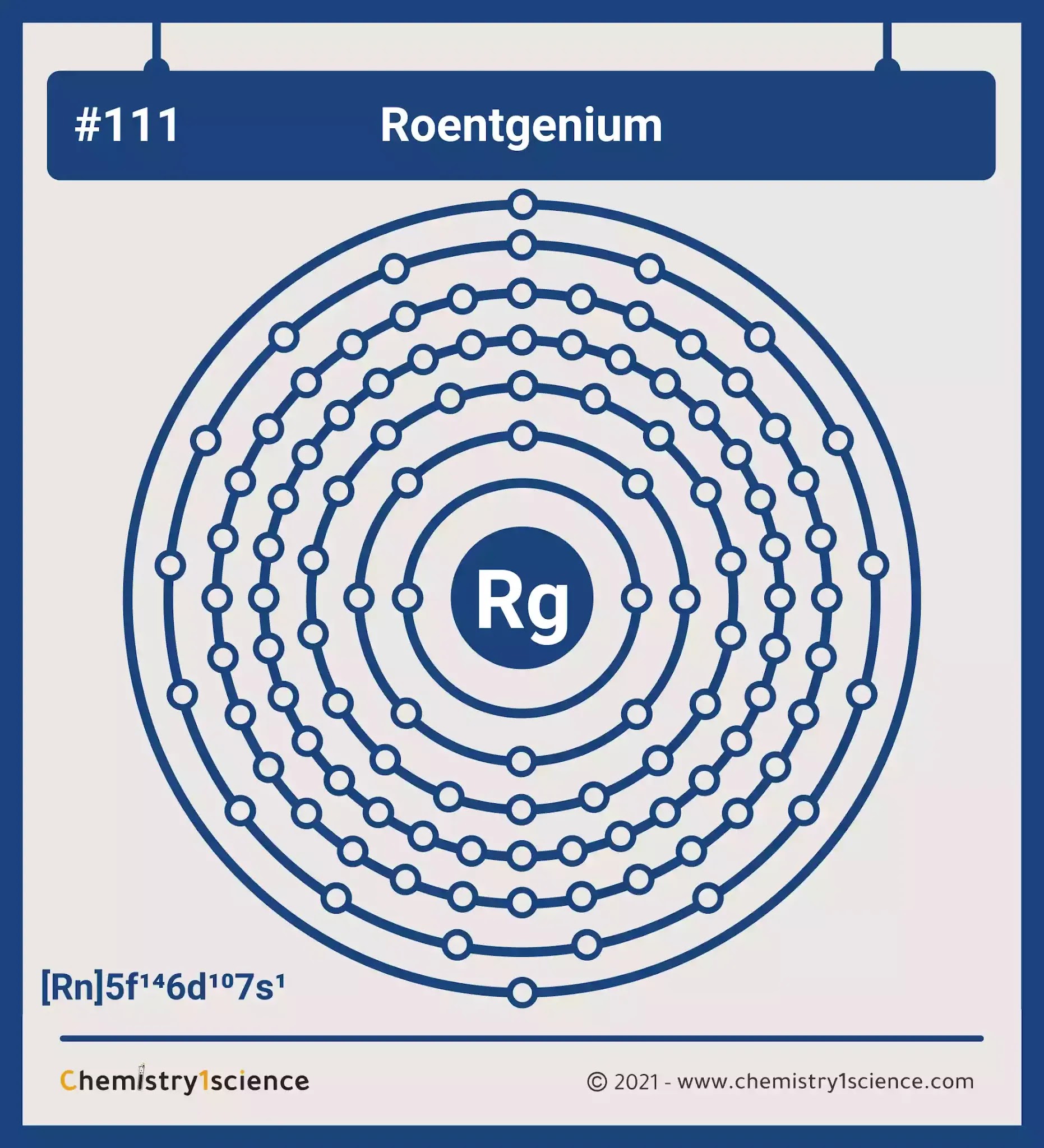
Atomic mass of Roentgenium most stable isotope 282 u.

. The symbol for molybdenum is Mo. There are seven known isotopes of the element. How many valence electrons does Sn 2 have.
Roentgenium is a chemical element with atomic number 111 which means there are 111 protons in its nucleusTotal number of protons in the nucleus is called the atomic number of the atom and is given the symbol ZThe total electrical charge of the nucleus is therefore Ze where e elementary charge equals to. Therefore the valence electrons of vanadium are five. Therefore a roentgenium atom will have two electrons in the first shell eight in the 2nd orbit eighteen electrons in the 3rd shell thirty-two in the 4th shell.
Valence electrons in Roentgenium Rg 11. In 1986 physicists at the Russian Joint Institute for Nuclear Research JINR bombarded bismuth with nickel hoping to make element 111 but they failed to detect any atoms of element 111. The Group number that element is in will tell you how many electrons the element has.
The electronic configuration of selenium atom is 4s 2 3d 10 4p 4. For neutral atoms the number of valence electrons is equal to the atoms main group number. Of course you have you are doing your chemistry homework.
Telluriums properties are intermediate between those of metals and nonmetals making it a metalloid. The atomic number is the number of electrons in that element. 111 Rg - Roentgenium.
Valence electrons in Nihonium Nh 3. Depending on your level of Chemistry it is probably easier to think of them as particles. Electrons arrangement in Roentgenium or Bohr model of Roentgenium.
Pick a picture of a Periodic. 112 Cn - Copernicium. Arsenic is a Main-group element so a quick simplification can be used.
So there are six electrons in the valence shell of the selenium. Its atomic number is 11 so it has 11 protons. Here is the trick.
For example carbon is in group 4 and has 4 valence electrons. 4f14 5d10 6s2 6p3. That is the number of electrons in roentgenium is one hundred eleven.
Neutrons in Roentgenium. Now lets check the facts about Astatine. The main group number for an element can be found from its column on the periodic table.
Atomic hatchet Symbol Quantum Numbers 4S32. How many protons neutrons and electrons are in a roentgenium atom. Roentgenium has 111 protons an 111 electrons.
In our example since carbon is in group 14 we can say that one atom of carbon has four valence electrons. Roentgenium has 111 protons an 111 electrons. Electronic configuration of Roentgenium Rn 5f 14 6d 9 7s 2.
Gallium Group 13 has 3 valence electrons. As noted above the elements in groups 3 to 12 are called transition metals and behave differently than the rest of the elements when it comes to valence electrons. Therefore the number of electrons in neutral atom of Roentgenium is 111.
Oxygen is in group 6 and has 6 valence electrons. Having six valence electrons tellurium tends to hold on to its electrons rather tightly. Electrons and Electron Configuration.
The electron configuration of vanadium shows that the last shell of vanadium has two electrons and the d-orbital has a total of three electrons3d 3. Nevertheless the electron affinity of roentgenium is expected to be around 16 eV 37 kcalmol significantly lower than golds value of 23 eV 53 kcalmol so roentgenides may not be stable or even possible. The atomic number of molybdenum is 42 and its electron configuration is 1s22s22p63s23p63d104s24p64d55s1 or 2 8 18 13 1 electrons per shell.
Gold also forms a somewhat stable 1 state due to relativistic effects and it has been suggested roentgenium may do so as well. Fluorine Group 17 has 7 valence electrons. Atoms are neutral so this means sodium also has 11 electrons.
Selenium Group 16 has 6 valence electrons. Electrons are arranged in shells or energy levels. Atomic Number Protons Electrons and Neutrons in Roentgenium.
For main group elements ie s-block and p-block elements the valence electrons are the electrons present in the outermost orbit. Tellurium has a total of six valence electrons. We have shown the Valence Electrons of the elements for which reliable data is available.
Each electron is influenced by the electric fields produced by the positive nuclear charge and the other Z 1 negative electrons in the atom. Valence electrons in Flerovium Fl 4. How many protons neutrons and electrons are in a roentgenium atom.
Number of electrons with no charge 83. The electrons in the 4d55s1 constitute its valence electrons. In the case of Astatine the valence electrons is 1357.
On the periodic table tellurium sits in the sixth row below selenium. It is classified as a transition metal and placed in group 6 or d-block and period 5 of the periodic table of elements. Number of neutrons Atomic mass of a given isotope of Rg - 111.
Valence electrons in Copernicium Cn 12. Astatine Overview Astatine Valence Electrons 1357 Atomic Number 85. Oxidation State-3-2-1 12345.
2 8 18 32 32 17 2. 272 274 and 278-282. Got a Periodic Table.
The atomic number of roentgenium is 111. All you need is a Periodic table. Number of neutrons Atomic mass of a given isotope of Rg - 111.
Find an element from Groups 3 to 12. Fluorine and selenium tend to be oxidizing and form ions of what charge. Gallium a metal tends to form positive ions of what charge.
It is a non-metal and present in the p block of the periodic table. Valence electrons are the outermost electrons and are the ones involved in bondingSodium has 11 electrons. The longest lived is isotope 281 which has a half-life of 228 seconds.
Shell structure Electrons per power level 2 8 18 32 18 5 Electron configuration. Ok but how many valence electrons does an atom of Astatine have. Crystal structure of Roentgenium.
The number of electrons in an electrically-neutral atom is the same as the number of protons in the nucleus. Valence Electrons Graph - Valence Electrons of all the elements in graph.

Roentgenium Wikipedia Bahasa Indonesia Ensiklopedia Bebas
Roentgenium Electron Configuration Symbol Atomic Number Atomic Mass Oxidation States Standard State Group Block Year Discovered

Roentgenium Facts Symbol Discovery Properties And Uses

Roentgenium Facts Symbol Discovery Properties And Uses
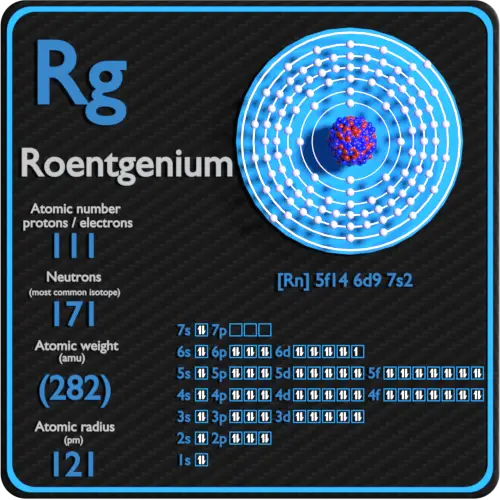
Roentgenium Protons Neutrons Electrons Electron Configuration

Roentgenium Atomic Structure Stock Image C024 8611 Science Photo Library
Hassium Atomic Structure Has Atomic Number Stock Vector Royalty Free 1920753038

Rutherfordium Art Print By Carlos Clarivan Electron Configuration Atomic Structure Gold Art Print
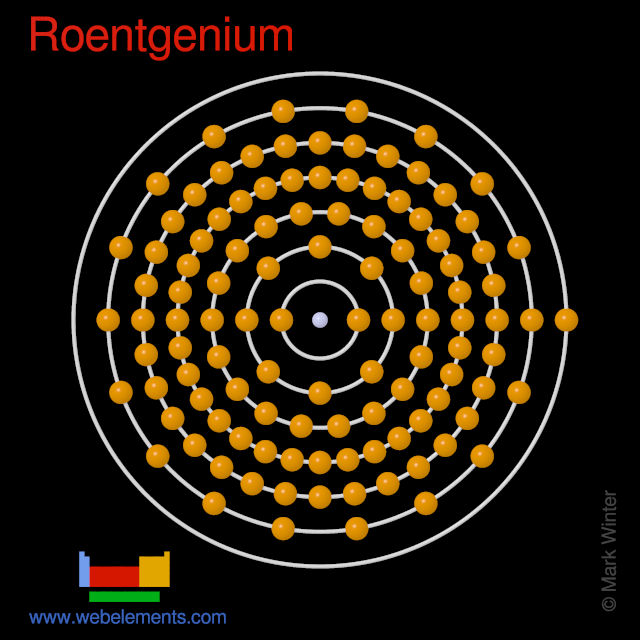
Webelements Periodic Table Roentgenium Properties Of Free Atoms
0 Comments